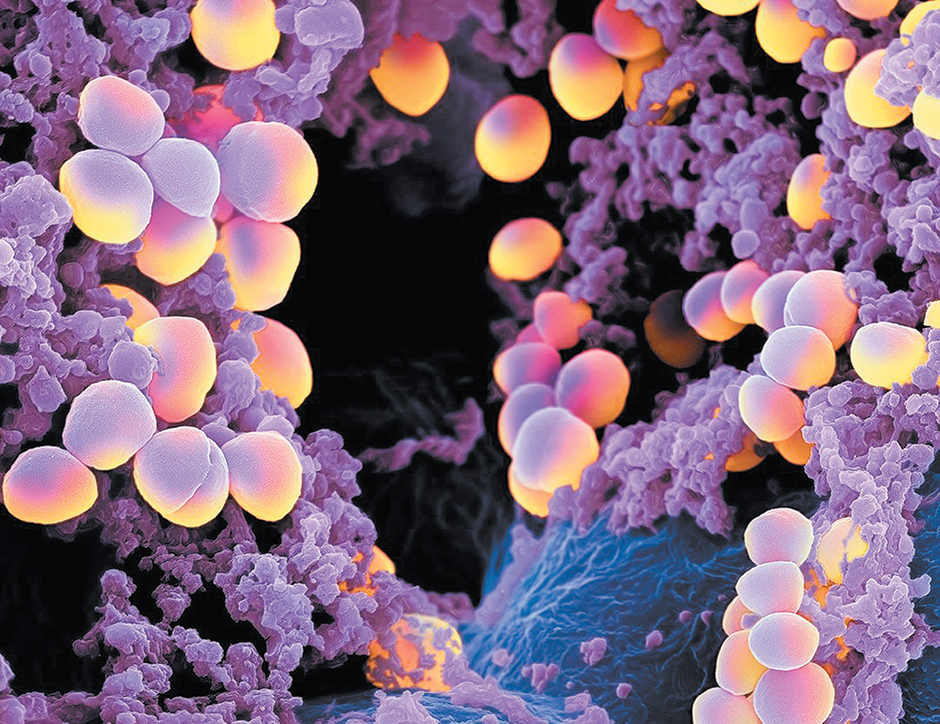
Martin Oeggerli/Micronaut
A colorized image, made with a scanning electron microscope, of Staphylococcus aureus bacteria. According to a recent National Geographic feature on microbes, Staphylococcus aureus ‘lives harmlessly in the noses of about a third of us. But it can turn rogue, causing skin infections—or worse.’
In 1609 Galileo Galilei turned his gaze, magnified twentyfold by lenses of Dutch design, toward the heavens, touching off a revolution in human thought. A decade later those same lenses delivered the possibility of a second revolution, when Galileo discovered that by inverting their order he could magnify the very small. For the first time in human history, it lay in our power to see the building blocks of bodies, the causes of diseases, and the mechanism of reproduction. Yet according to Paul Falkowski’s Life’s Engines:
Galileo did not seem to have much interest in what he saw with his inverted telescope. He appears to have made little attempt to understand, let alone interpret, the smallest objects he could observe.
Bewitched by the moons of Saturn and their challenge to the heliocentric model of the universe, Galileo ignored the possibility that the magnified fleas he drew might have anything to do with the plague then ravaging Italy. And so for three centuries more, one of the cruellest of human afflictions would rage on, misunderstood and thus unpreventable, taking the lives of countless millions.
Perhaps it’s fundamentally human both to be awed by the things we look up to and to pass over those we look down on. If so, it’s a tendency that has repeatedly frustrated human progress. Half a century after Galileo looked into his “inverted telescope,” the pioneers of microscopy Antonie van Leeuwenhoek and Robert Hooke revealed that a Lilliputian universe existed all around and even inside us. But neither of them had students, and their researches ended in another false dawn for microscopy. It was not until the middle of the nineteenth century, when German manufacturers began producing superior instruments, that the discovery of the very small began to alter science in fundamental ways.
Today, driven by ongoing technological innovations, the exploration of the “nanoverse,” as the realm of the minuscule is often termed, continues to gather pace. One of the field’s greatest pioneers is Paul Falkowski, a biological oceanographer who has spent much of his scientific career working at the intersection of physics, chemistry, and biology. His book Life’s Engines: How Microbes Made Earth Habitable focuses on one of the most astonishing discoveries of the twentieth century—that our cells are comprised of a series of highly sophisticated “little engines” or nanomachines that carry out life’s vital functions. It is a work full of surprises, arguing for example that all of life’s most important innovations were in existence by around 3.5 billion years ago—less than a billion years after Earth formed, and a period at which our planet was largely hostile to living things. How such mind-bending complexity could have evolved at such an early stage, and in such a hostile environment, has forced a fundamental reconsideration of the origins of life itself.
At a personal level, Falkowski’s work is also challenging. We are used to thinking of ourselves as composed of billions of cells, but Falkowski points out that we also consist of trillions of electrochemical machines that somehow coordinate their intricate activities in ways that allow our bodies and minds to function with the required reliability and precision. As we contemplate the evolution and maintenance of this complexity, wonder grows to near incredulity.
One of the most ancient of Falkowski’s biological machines is the ribosome, a combination of proteins and nucleic acids that causes protein synthesis. It is an entity so tiny that even with an electron microscope, it is hard to see it. As many as 400 million ribosomes could fit in a single period at the end of a sentence printed in The New York Review. Only with the advent of synchrotrons—machines that accelerate the movements of particles, and can be used to create very powerful X-rays—have its workings been revealed. Ribosomes use the instructions embedded in our genetic code to make complex proteins such as those found in our muscles and other organs. The manufacture of these proteins is not a straightforward process. The ribosomes have no direct contact with our DNA, so must act by reading messenger RNA, molecules that convey genetic information from the DNA. Ribosomes consist of two major complexes that work like a pair of gears: they move over the RNA, and attach amino acids to the emerging protein.
All ribosomes—whether in the most humble bacteria or in human bodies—operate at the same rate, adding just ten to twenty amino acids per second to the growing protein string. And so are our bodies built up by tiny mechanistic operations, one protein at a time, until that stupendous entity we call a human being is complete. All living things possess ribosomes, so these complex micromachines must have existed in the common ancestor of all life. Perhaps their development marks the spark of life itself. But just when they first evolved, and how they came into being, remain two of the great mysteries of science.
Advertisement
All machines require a source of energy to operate, and the energy to run not only ribosomes but all cellular functions comes from the same source—a universal “energy currency” molecule known as adenosine triphosphate (ATP). In animals and plants ATP is manufactured in special cellular structures known as mitochondria. The nanomachines that operate within the mitochondria are minute biological electrical motors that, in a striking parallel with their mechanical counterparts, possess rotors, stators, and rotating catalytic heads.
The ATP nanomachine is the means by which life uses electrical gradients, or the difference in ion concentration and electrical potential from one point to another, to create energy. The nanomachine is located in a membrane that separates a region of the cell with a high density of protons (hydrogen ions) from an area with a lower density. Just as in a battery, the protons pass from the area of high density into the area of lower density. But in order to do so in the cell, they must pass through the ATP nanomachine, and their flow through the minute electric motor turns its rotor counterclockwise. For every 360-degree turn the rotor makes, three molecules of ATP are created.
Living things use a great many primary energy sources to create ATP. The most primitive living entities are known as archaea. Though bacteria-like, they are a distinct group whose various members seem to have exploited almost every energy source available on the early Earth. Some, known as methanogens, cause carbon dioxide to react with hydrogen to create the electrochemical gradient required to make ATP, producing methane as a by-product. Others use ammonia, metal ions, or hydrogen gas to create the electrochemical gradient. Bacteria also use a variety of energy sources, but at some point a group of bacteria started to use sunlight to power photosynthesis. This process yielded vastly more energy than other sources, giving its possessors a huge evolutionary advantage. Falkowski has spent most of his career unraveling the deep mystery of photosynthesis and how it changed the world.
He calls the photosynthetic process “almost magical.” His description gives a flavor of the magic involved:
When one, very specific chlorophyll molecule embedded in a reaction center absorbs the energy from a photon, the energy of the light particle can push an electron off the chlorophyll molecule. For about a billionth of a second, the chlorophyll molecule becomes positively charged.
The electron “hole” in the chlorophyll molecule is in turn filled by an electron from “a quartet of manganese [the chemical element] atoms held in a special arrangement on one side of a membrane.” The electron “hole” thus formed in the manganese quartet is filled with electrons from a water molecule. This causes the water molecule to fall apart, creating free oxygen.
Photosynthesis permits a local and temporary reversal of the second law of thermodynamics—the creation of order out of disorder. Magical indeed, but in early 2014 photosynthesis was revealed to be even more magical than Falkowski’s book allows. Physicists based in the United Kingdom demonstrated that quantum mechanics plays a vital part in the photosynthetic process, by helping to transport the energy it captures efficiently, in a wavelike manner.1
If chemistry is not your cup of tea, Falkowski offers an alternative way of thinking about how photosynthesis works—as a microscopic sound and light show. The light is of course the photon that energizes the performance, while the sound is provided by the chlorophyll molecule, which flexes with an audible “pop” when it loses its electron. The phenomenon was discovered by Alexander Graham Bell, who in 1880 used what he called the “photoacoustic effect” to make a device he named the photophone. Bell used the photophone to transmit a wireless voice telephone message seven hundred feet, and considered it to be his greatest invention. And perhaps it was, since it was the precursor of fiber optic communication.
The way that the sophisticated nanomachines Falkowski describes became incorporated into a single complex cell, such as those our bodies consist of, is so incredible that it reads like a fairy tale. Using a system known as “quorum sensing,” microbes can communicate, and they use this ability to switch on and off various functions within their own populations and within ecosystems composed of different microbe species. Quorum sensing can even operate when one microbe swallows another, as happened over a billion years ago when a larger cell began to communicate with a smaller one that it had ingested. Quorum sensing permitted the potential food item to live inside its host instead of being digested. Then it allowed genes to switch on and off in ways that benefited the new chimeric, or genetically mixed, entity. The two genomes coexisting in the chimera even managed to exchange some genes, further enabling it to operate as a competent whole. As a result of these changes, the organism that was swallowed was transformed into a mitochondria, and began supplying ATP to the first eukaryotic cell—that is, a cell containing a nucleus and other complicated structures.
Advertisement
As impossible as this process sounds, it was followed by an even more outlandish occurrence. Somehow the newly created binary organism swallowed yet another entity—a kind of bacteria that could photosynthesize. Again the ingested entity lived on inside the cell, using quorum sensing to somehow synchronize its “almost magical” nanomachinery with those of the binary organism. This newly constituted “trinity organism” became the photosynthetic ancestor of every plant on earth.
Microbes control the Earth, Falkowski tells us. They created it in its present form, and maintain it in its current state by creating a global electron marketplace that we call the biosphere. Falkowski argues that we can conceive of our world as a great, unitary electrical device, driven by the myriad tiny electric motors and the other electrochemical nanomachinery of cells. Viewing the world this way reveals hitherto unappreciated dangers in some modern science.
Some molecular biologists are doing research on ways of inserting genes into microorganisms in order to create new kinds of life that have never previously existed. Others are busy working out whether the cellular nanomachinery itself might be improved. Falkowski recommends that
rather than tinker with organisms that we can’t reverse engineer, a much better use of our intellectual abilities and technological capabilities would be to better understand how the core nanomachines evolved and how these machines spread across the planet to become the engines of life.
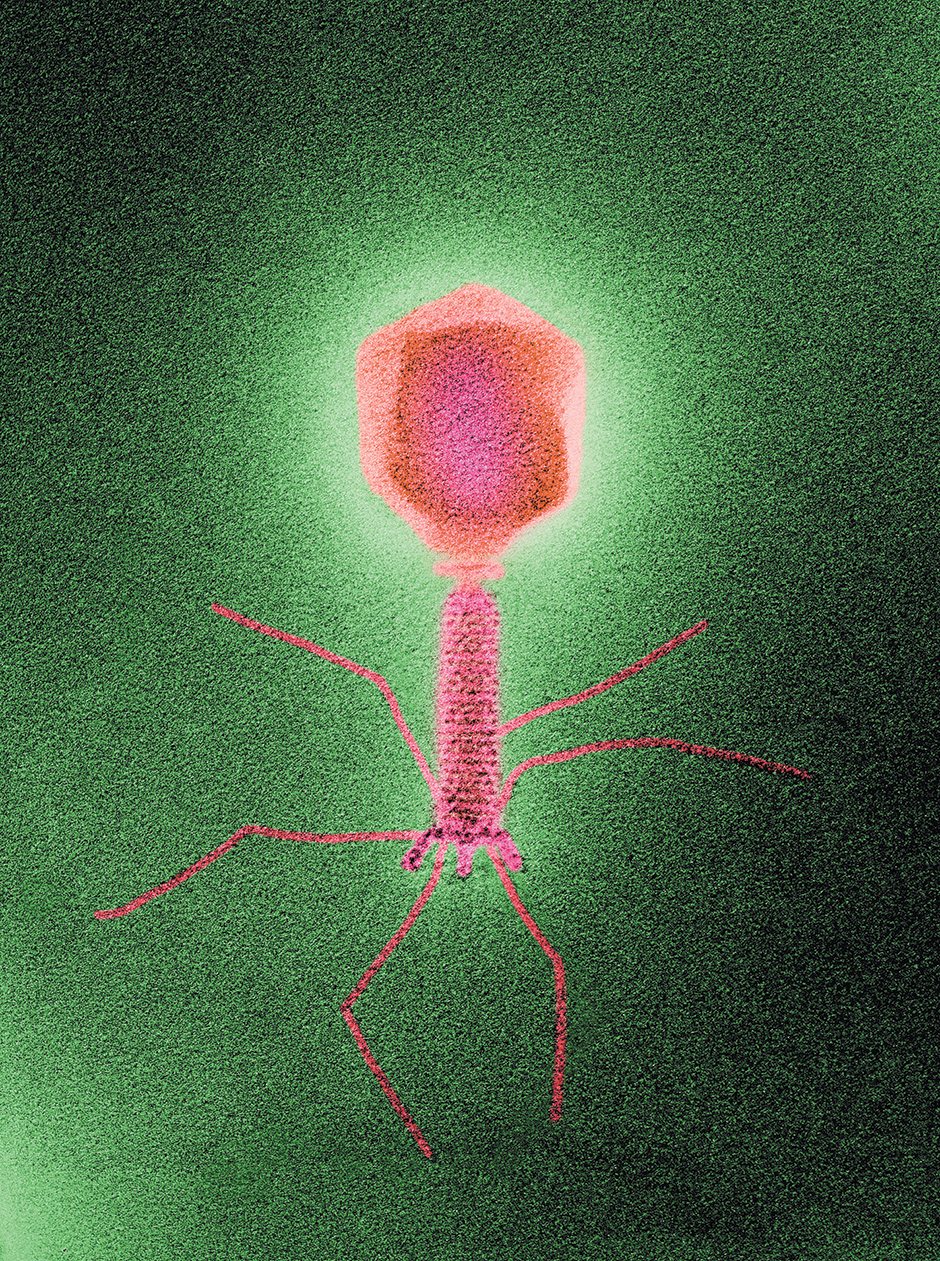
Biozentrum, University of Basel/Science Source
A colored transmission electron micrograph of a bacteria-infecting virus, or phage. According to National Geographic, phages ‘are the most abundant life-form on the planet, their number far exceeding that of stars in the universe. Trillions inhabit each of us.’
Just how far we are from obtaining an understanding of the evolution of the nanomachines is conveyed in Peter Ward and Joe Kirschvink’s latest book, A New History of Life. Both authors are iconoclasts, and their book is at times breathtakingly unorthodox. Yet their ideas are at the cutting edge of many debates about the evolution of life, making their book challenging and rewarding. The work of the paleontologist is like that of a restorer of ancient mosaics: the further we go back in time, the fewer tesserae, or mosaic components, we have. Those seeking to understand the origin of the nanomachines have to work with the equivalent of just half a dozen pieces from a picture comprising tens of thousands. Time and our restless Earth have destroyed the remainder. Despite this awesome handicap, Ward and Kirschvink are convinced that, owing to the new technologies, we are at last asking the right questions.
We have a reasonably concise date for the formation of Earth—4,560 million years ago, give or take 10 million years. The half a billion years that followed, known as the Hadean Eon, were momentous. A huge asteroid slammed into the planet, forming the moon and transforming Earth into a ball of molten rock. As Earth cooled, the progenitors of the modern core and crust were formed. Earth’s oldest rocks—tiny, 4.4-billion-year-old zircon crystals from Western Australia—are the only physical evidence we have of this period. Chemical analysis reveals that they formed where ocean water was being sucked down into the mantle—the layer of the earth between the crust and the core. So we can surmise that Earth cooled quickly after the asteroid collision, and that at an early stage it had oceans.
Despite the presence of oceans, Earth was almost certainly hostile to life in the Hadean Eon. Asteroid impacts repeatedly shook the planet, boiling its oceans and changing the atmosphere. But by four billion years ago, things had begun to settle down. The 1.5-billion-year-long Archean Eon had begun, and it was over the first third of this period that the nanomachines either evolved, or as Ward and Kirschvink argue, colonized Earth from elsewhere.
As we ponder life’s origins, Ward and Kirschvink warn against thinking in simplistic terms like life and death, instead encouraging us to consider the “newly discovered place in between.” Life’s most distant origins lie in the nonliving precursor molecules for RNA, organic compounds known as amino acids. They have been found in meteorites, are presumed to be widespread in the universe, and their origins must greatly predate Earth’s origins. The nanomachines possess attributes of life, and when brought together in a cell they clearly cross the threshold into the self-regulating, replicating entity that we recognize as a living thing.
A slick layer of graphite preserved in 3.8-billion-year-old rocks near Isua, Greenland, was long believed to contain the earliest evidence of life on Earth. But recent studies reveal that the carbon composing the graphite was not formed by life at all. The next oldest evidence was long thought to be 3.5-billion-year-old microscopic fossils of algae from Western Australia. But recent research has shown that the “fossils” are far more recent, and in any case may not be fossils at all, but crystals. A 2012 study announced that fossils of bacterial ecosystems dating back 3.49 billion years had been discovered in the Pilbara region of Western Australia, and this is now widely accepted as the oldest evidence of life.2
Charles Darwin famously speculated that life began in a “shallow, sun-warmed pond.” But back when Earth formed, its surface was probably covered entirely, or almost so, by oceans. And because Earth lacked an ozone layer for the first two billion years of its existence, it is unlikely that shallow waters could have hosted life’s origin because ultraviolet radiation would have torn apart the delicate, assembling RNA.
Currently favored candidates for an earthly origin of life range from hot springs to mid-ocean ridge vents known as “black smokers.” Conditions there may have aided the formation of the ever-longer strings of amino acids and molecules, including RNA, that were eventually able to metabolize and reproduce. Mid-oceanic ridges are protected from ultraviolet radiation by the overlying ocean. They are also rich in the elements required for DNA. Additionally, the majority of the most ancient life forms on Earth are thermophiles, small organisms some of which thrive in near-boiling water. One problem for this theory is that water attacks and breaks up the nucleic acid polymers that make up RNA. And unless protected, it is also destabilized by heat.
Most research focuses on a search for the earliest life. But perhaps we should be searching instead for evidence of the first nanomachines. Chemical signatures in rocks that result from the activities of the nanomachines offer one means of doing this. For example, studies show that the nanomachines that make atmospheric nitrogen, and can add oxygen to the ammonia so produced in order to create nitrate, were in existence by at least 2.5 billion years ago.3
Joe Kirschvink argues that Earth’s rocks are the wrong place to look for the nanomachines’ origins. He is a leading proponent of the seemingly radical theory that the nanomachines, and perhaps life itself, originated on the ice caps and glaciers of ancient Mars. The case is fleshed out fully in A New History of Life, and recent discoveries are building an impressive body of supporting evidence. NASA’s Curiosity lander, for example, has found evidence for ancient Martian streams and ponds: billions of years ago Mars probably had an ocean, as well as land and ice caps. The red planet may have offered a far less hostile environment for assembling naked strings of RNA than Earth. Kirschvink also points out that space travel by early life is not improbable. Mars is small, so its gravity is weak compared with that of Earth. Asteroids could therefore have thrown up a lot of rocks capable of escaping Martian gravity. And we know, through experiments, that meteorites originating from Mars can reach Earth without being sterilized.
But if the nanomachines did originate on Mars, where might they have crossed the “Darwinian threshold” and become truly living things? Kirschvink argues that Earth’s atmosphere offers a plausible nursery. Held aloft by fierce winds and currents, the Martian RNA fragments may have mixed with each other, exchanging fragments from one chain to another. Natural selection would have favored the more functionally complex and efficient strands, which would then have proliferated. Eventually, perhaps when the strands became encompassed by cell walls made of tiny droplets of lipids (a type of molecule that includes fats and waxes), the mass transfer of genes between the nascent nanomachines slowed and their chemistry stabilized.
The Nobel laureate Christian de Duve believed that at this point life would have emerged from nonlife very quickly, perhaps in minutes. Safe behind its lipid cell walls, the RNA could enter the ocean, finding the rich trove of nutrients that exists around the black smokers. From then on, Darwinian evolution would have ensured the survival of those that operated most efficiently in a hot environment. This story is, of course, almost entirely unsupported by evidence. It is a scenario—a vision of how things might have been—rather than a fleshed-out scientific theory. It is nonetheless useful because it provides a target for future researchers.
A New History of Life deals with life’s entire trajectory, from the time before its first spark to the present. The conventional view is that for a billion years after life first evolved, very little seems to have happened. Then, over perhaps a few hundred million years, oxygen utterly transformed the face of Earth. That oxygen came from the most complex cellular nanomachinery ever to evolve—the trinity organisms, composed of three organisms embedded within a single cell, that could photosynthesize. But Falkowski’s nanomachines make me think that the billion-year “pause” before their emergence is illusory. Enormous changes to life’s engines occurred as they transformed from relatively simple nanomachines to planet-altering photosynthesizers.
A mystery surrounds the oxygenation of Earth. The oxygen produced by the photosynthesizers should have interacted immediately with organic matter, preventing any increases in free atmospheric oxygen. And indeed this is what appears to have happened for hundreds of millions of years after the first trinity organisms evolved. What was needed, if free oxygen was to accumulate in the atmosphere, was for some of the organic matter it reacted with to be put out of the oxygen’s reach.
Falkowski thinks that “the oxygenation of Earth had much to do with chance and contingencies.” Ward and Kirschvink agree, saying that one of the greatest contingencies was the creation of what we call fossil fuels. For fossil fuels and other buried organic molecules are organic matter put out of oxygen’s reach many millions of years ago, and they exist in Earth’s crust in direct proportion to the amount of oxygen in the atmosphere.
The dependence of evolutionary change on contingencies is further highlighted when Ward and Kirschvink discuss the evolution of the first large animals. They arose about half a billion years ago, in what is known as the Cambrian explosion. Scientists have long argued about why they evolved so rapidly, and at that time. Ward and Kirschvink think they have an answer, in the form of “true polar wander.” Essentially, the idea is that as the continents moved over the face of the planet, they altered its center of gravity. By around half a billion years ago they had so shifted the gravitational center that the Earth’s outer layers had begun to move relative to Earth’s core. Over millions of years, the landmasses originally lying over the poles came to lie over the equator. This southward shift may have released methane trapped in clathrates (ice-methane combinations kept stable by low temperatures or pressure), triggering a release of greenhouse gases that warmed the climate and provided favorable conditions for an increase in biodiversity. There is evidence to back parts of this theory. Something odd was happening to Earth’s poles around the time complex life evolved. And “true polar wander” is characteristic of other planets, including Mars. But again, Ward and Kirschvink are pushing the envelope with this theory.
Neither Life’s Engines nor A New History of Life is an easy book for the nonscientist, but both are immensely rewarding. Like Galileo’s telescope and microscope, they focus on the very small (Falkowski) and the very big picture (Ward and Kirschvink). Both are full of novel thinking about life’s origin and subsequent evolution. Taken together, they help us begin to see where the next big questions about life’s origins lie, and how they might be investigated.
-
1
See Jon Cartwright, “Quantized Vibrations Are Essential to Photosynthesis, Say Physicists,” physicsworld.com, January 22, 2014. ↩
-
2
See Nora Noffke, Daniel Christian, David Wacey, and Robert M. Hazan, “A Microbial Ecosystem in an Ancient Sabkha of the 3.49 GA Pilbara, Western Australia, and Comparison with Mesoarchean, Neoproterozoic and Phanerozoic Examples,” GSA Annual Meeting, November 2012. ↩
-
3
See “Billions of Years Ago, Microbes Were Key in Developing Modern Nitrogen Cycle,” (e) Science News, February 19, 2009. ↩



