In a major decision issued on June 13, the US Supreme Court unanimously struck down the patents held by a biotechnology firm on the DNA comprising BRCA1 and BRCA2.1 These are the two genes that, in their abnormal forms, are known to dispose women to a dramatically heightened risk of breast and/or ovarian cancer. The case had originated in May 2009, when the American Civil Liberties Union and the Public Patent Foundation filed a lawsuit in a federal district court to overturn the patents. The main defendant was the Myriad Genetics Corporation in Utah, which had located the two genes, extracted them from the chromosomes housing them, and, in 1997 and 1998, obtained the patents in question. The patents did not cover the DNA of the two genes while they are in the body but only in isolation from it.2
The patents controlled by Myriad entitled the company to exclude all others from using the isolated DNA in breast cancer research, diagnostics, and treatment. The plaintiffs—who originally included biomedical scientists and clinicians, advocates for women’s health, and several women with or at risk for breast cancer—held that Myriad’s enforcement of its patents interfered with the progress of science and the delivery of medical services. They contended that genes, even if isolated, were legally ineligible for patents and that well-established tenets of patent law precluded the grant to any person or institution of a monopoly over a substance so essential to life, health, and science as human DNA.
In March 2010, the district court struck down Myriad’s DNA patents, but in July 2011 the Court of Appeals for the Federal Circuit upheld them. After being told by the Supreme Court to vacate its finding, the appeals court upheld the patents again in August 2012. It had denied standing in the case to all the plaintiffs except one, Harry Ostrer, a biomedical scientist then at NYU. On November 30, 2012, the Supreme Court agreed to review the case, stipulating that both Ostrer and Myriad address only the salient issue of whether genes were eligible for patents.
A section of the US Code spells out the relevant criterion of eligibility for the patents in dispute. They can be granted to “whoever invents or discovers any new and useful…composition of matter.” Since the later nineteenth century, the courts have excluded from eligibility for patents laws of nature, natural phenomena, and natural products—for example, trees in the field and minerals in the earth. They were, the courts said, not new; they existed without anyone having made “an inventive step,” and universal access to them was essential to the progress of science and invention. They were, as the Supreme Court had said in a decision in 1948, “free to all men and reserved exclusively to none.” However, the courts had also held that products of nature could qualify for a patent if an inventive step gave them—to quote the Court’s 1980 ruling in Diamond v. Chakrabarty, a landmark case in the patenting of life—“markedly different characteristics from any found in nature.” The life in question consisted of genetically modified bacteria that were capable of breaking down crude oil.
In March 2012, in the case of Mayo Collaborative Services v. Prometheus Laboratories, the Court had unanimously reaffirmed these precedents, striking down a patent that covered a law of nature. Delivering the Court’s opinion, Justice Stephen Breyer noted the repeated emphasis in its decisions that a patent required an “inventive concept” and “that patent law not inhibit further discovery” or “impede innovation more than it would tend to promote it” by granting monopolies over laws of nature, manifestations of nature, and natural phenomena.
The core issue in the gene patents case was thus whether the isolated DNA qualified for a patent because it was a new composition of matter that Myriad had invented or was disqualified because it was an unmodified product of nature, the control of which through a patent obstructed the progress of science and invention.
Some fifty friend-of-the-court briefs were filed on behalf of Ostrer, Myriad, and neither party. The Court’s chamber was jammed at the oral arguments on April 15. Popular interest in the case rose when on May 14 Angelina Jolie, the award-winning actress and film director, announced in The New York Times that, coming from a family with a high incidence of breast cancer, she had been tested for BRCA, learned that she possessed the abnormal form of BRCA1, and had undergone a preemptive double mastectomy. She declared that the test and surgery had enabled her to take control of her life, reducing her chances of breast cancer from 87 percent to under 5 percent. She urged women with a pattern of hereditary breast or ovarian cancer in their families to get tested. But she regretted that, at some $3,000, the test was too expensive for many women at risk.
Advertisement
Myriad’s patents covered two types of isolated DNA. One was the whole or a fragment of the BRCA DNA—“genomic DNA,” scientists say—as it was extracted intact from the body. Genomic DNA spans the sequence of base pairs—they are the rungs across the ladder of the double helix—some that code for amino acids and, interspersed among them, many more that do not. The former are called “exons,” the latter “introns.” The other type of isolated DNA is called “complementary DNA,” or cDNA, which comprises only the exons and is produced by scientists in the laboratory.
Gregory Castanias, who argued the case for Myriad, claimed that isolated genomic BRCA DNA was eligible for a patent on two grounds. First, Myriad’s scientists had taken a major inventive step in finding the gene and then deciding at what points to separate it from the DNA at its flanks. Second, by extracting it from the body they had broken its chemical connections to the flanking DNA and had thus created a new composition of matter useful for diagnostics and other purposes. Castanias said that cDNA was patent-eligible because it was created by scientists, not by nature.
Myriad’s lawyers emphasized that their arguments had the approval of the US Patent and Trademarks Office. The PTO had on its own decided in the early 1980s to begin issuing patents, including Myriad’s, on both types of isolated DNA and in January 2001 had for the first time publicly explained its position, citing among other points of law the “markedly different” criterion justifying the issuance of patents in the Chakrabarty case. Myriad’s lawyers insisted that the PTO, operating expertly at the intersection of law, science, and policy, was owed deference.
Christopher Hansen, of the ACLU, ably argued the case for Ostrer, and he was aided by the Department of Justice. Representing the United States, the department had filed a friend-of-the-court brief, and the solicitor general, who has rarely appeared in a patent suit, participated in the oral arguments. Although the brief was formally offered on behalf of neither party, it opposed the position of the PTO and in substance supported that of Ostrer. While Hansen laid out the case against Myriad’s patents in its particulars, the Justice Department focused on the principles of it, making a forceful combination.
Hansen opened the oral arguments with the question: “What exactly did Myriad invent?” and then declared, “Nothing.” Myriad deserved credit for finding the BRCA genes, but such scientific accomplishments, he said, were not eligible for patents. The DNA it isolated was not markedly different; it was unchanged in its genetic information from the DNA in the body. The isolation of the DNA, routine in science at the time, did not derive from an inventive concept. Myriad might claim that the isolated DNA gained new uses outside the body, but that did not make it a patentable invention.
Why not? Justice Samuel Alito asked, given that in isolation it acquired a new function. Hansen responded that even if you found a new use for a product of nature, it was not patent-eligible if you did not change it. “If I find a new way of taking gold and making earrings out of it, that doesn’t entitle me to a patent on gold.” The Justice Department lawyers emphasized that DNA extracted from the body was no more patent-eligible than many natural substances—for example, the pistils of the saffron flower or various naturally occurring elements such as lithium that had to be chemically isolated from its native mineral in order to be made useful.
The Justice Department lawyers declared that the PTO was not owed any deference in the case. It had the authority only to carry out law and policy, not to make either. Its gene-patenting practices had been sanctioned by neither Congress nor the courts. The ACLU and the Justice Department, however, disagreed over the patent-eligibility of cDNA. While Hansen contended that its ordered composition of exons was “dictated by nature,” the department’s lawyers considered it “the product of significant human creativity.”
The Court’s instruction in Mayo Collaborative Services the year before—that patents impeding innovation were impermissible—provided crucial leverage for the ACLU and the Justice Department lawyers. “Myriad’s monopoly on the BRCA genes,” Hansen pointed out,
has allowed it to dictate the quality and provision of BRCA genetic testing and to control the scientific knowledge about the genes, thereby limiting medical practice, chilling research, and restricting access to information crucial to women’s health.
Arguing the issue more generally, the Department of Justice found “useful guidance” in the Mayo decision for deciding the Myriad case, contending that a patent on a composition of matter
Advertisement
that effectively prevents the public from studying and using a product of nature is just as objectionable as a method claim that prevents the public from studying and exploiting a law of nature.
Patents on cDNA, because it differed from the natural product, posed no such risk.
During the oral arguments, the justices pressed Myriad’s lawyer Gregory Castanius hard on the natural products issue. A personal friend-of-the-court brief on behalf of neither party from Eric Lander had captured Justice Breyer’s attention. A leading geneticist, head of a world-class biomedical research institute at Harvard and MIT, and well experienced in biotechnology, Lander emphasized that chromosomes in the body were constantly being broken up into their constituent fragments of DNA, which included the BRCA genes. “Myriad’s claims thus include DNA fragments that are unambiguously products of Nature”—and were thus not patent-eligible.
Justice Breyer asked Castanias whether the Lander brief was wrong “as a matter of science,” adding with evident irritation after Castanias seemed to go off on a tangent, “I’d like a yes or no answer.” Castanias finally said that he disagreed with Lander’s science but did not say why, whereupon Breyer retorted, “I would like you to tell me what I should read that will, from a scientist, tell me that it’s wrong.” Castanias hesitated, then lamely told Breyer to consult one of the scientific documents in the Myriad case file.
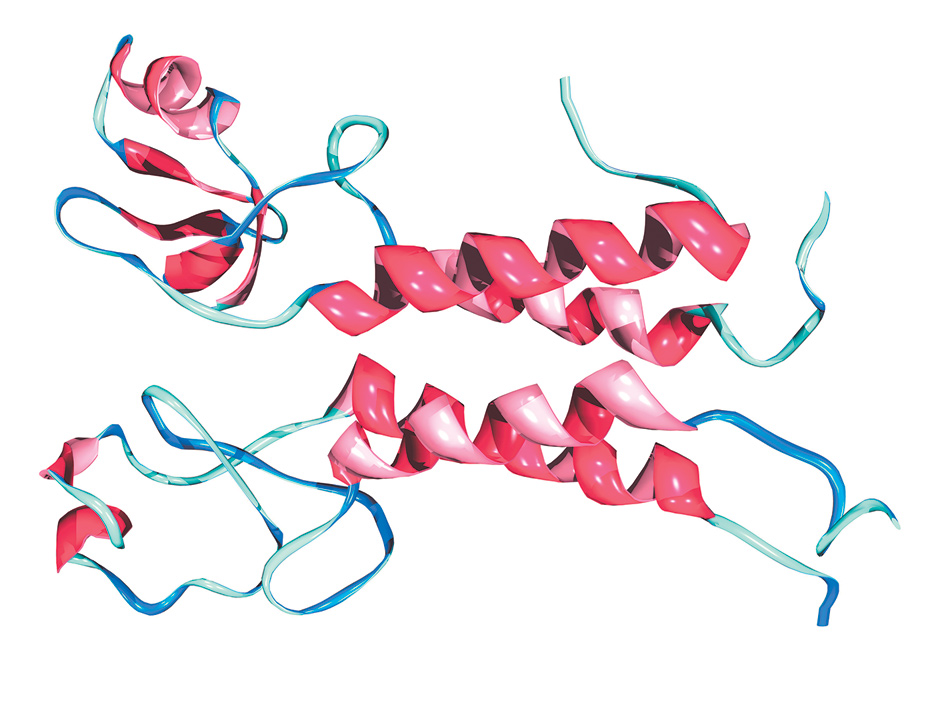
Dr. Mark J. Winter/Science Source
A structural representation of, at top, the BRCA1 protein, the molecule produced by the BRCA1 gene in breast and other tissues, and below it, a second protein called Bard1 with which it functions in partnership. The four red-pink spirals indicate sections in the two molecules in the form of a helix that hold them together; the blue-aqua ribbons represent chains of amino acids, with some of those on the left comprising the active part of the complex. When the BRCA1 protein is produced by the normal BRCA1 gene, the partnership helps cells repair damage to their DNA. If that protein is generated by the abnormal version of the gene, the partnership’s capacity for repair is diminished, making tumors more likely.
Several of the justices proposed analogies to Castanias in an effort to understand why DNA extracted from the body that was essentially unchanged should be eligible for a patent. Justice Elena Kagan fastened on a plant in the Amazon rain forest that might be medicinally valuable. If it were made useful by uprooting it and removing it from the forest, she asked, would it qualify for a patent? Castanias allowed that it would not, which prompted Kagan to remark, “I guess what you haven’t gotten me to understand is how this [the isolated gene] is different from that [the isolated plant].”
Chief Justice John Roberts talked of a baseball bat. If you snipped a piece of wood from the branch of a tree, you don’t “all of a sudden [have] a baseball bat,” he noted, adding, “You have to invent it.” But when Myriad’s scientists extracted the DNA from the body they did not have to invent anything. They only had to snip it out along a certain line.
A question from Justice Sonia Sotomayor prompted Castanias to say that you could not get a patent on a cutting from the liver or a kidney. It was not the same thing as isolated DNA. “So what’s the difference?” Justice Sotomayor asked.
If you cut off a piece of the whole in the kidney or liver, you’re saying that’s not patentable, but you take a gene and snip off a piece, that is? What’s the difference between the two?
Justice Clarence Thomas, silent during the oral arguments, delivered the Court’s opinion, holding:
A naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated, but cDNA is patent eligible because it is not naturally occurring.
Myriad found and isolated the two genes but “did not create anything,” certainly not a new composition of matter. The grant of patents on products of nature such as isolated DNA would improperly tie up basic tools of science and technology. The composition of cDNA might be dictated by nature, but it was new and produced by human action. By contrast, the PTO’s long-standing practice of awarding patents on isolated genomic DNA was not entitled to deference, given that Congress had not endorsed the practice and that the United States, represented by the Justice Department, had weighed in against it.
The Court’s decision was applauded in many quarters, including a respected public radio station in Southern California that headlined the liberation of “Angelina Jolie’s breast cancer gene” from the clutch of patents. Harry Ostrer found the ruling “thrilling,” and Mary-Claire King, who had discovered BRCA1 when she was a professor at UC Berkeley, called it “a fabulous result for patients, physicians, scientists, and common sense.” Myriad, whose stock dropped some 25 percent in the week after the ruling, was clearly disappointed, as were many members of the biotechnology industry and the patent bar. But all could take some comfort from the fact that the Court had upheld the patent eligibility of cDNA.
The case may bespeak a fundamental change in biomedical patenting, suggesting that interested parties outside the biotechnology industry and the patent bar—scientists, patients, physicians, and health advocates—expect and would act to achieve standing in decisions concerning control of genomic DNA in biomedical research and practice.3 The Court’s opinion itself, while a constrained statement of fact and law, was also rich with consequences and implications. It appeared to break Myriad’s monopoly over BRCA research and testing, and several companies announced that they would soon offer BRCA tests far more cheaply.4
In effect, the opinion placed unmodified genomic DNA in the same category as the naturally occurring elements—unpatentable products of nature that had been discovered nevertheless and had been used to create innumerable patented inventions. It also could be taken to imply that other isolated but essentially unchanged bodily products—e.g., antibodies, hormones, and cells—may not be patent-eligible.5
The opinion certainly reminded the PTO and its clients that the agency was not a law unto itself. It had to take into account whether its decisions would allow continued universal access to natural laws and natural substances—in short, to what should be freely available to everyone and reserved exclusively to none.
This Issue
September 26, 2013
The American Jewish Cocoon
They’re Taking Over!
Stranglehold on Washington
-
1
US Supreme Court, Association for Molecular Pathology et al. v. Myriad Genetics, Inc., et al., June 13, 2013. ↩
-
2
For the background and development of the case, see Daniel J. Kevles, “Can They Patent Your Genes?,” The New York Review, March 7, 2013. All case documents are available through WestlawNext at store.westlaw.com/westlawnext. ↩
-
3
Castanias, sensitive but unsympathetic to the meaning of the case, disparaged the “abstract, hypothetical nature of this lawyer-engineered ‘test case.’” Brief for the Respondents, p. 46. ↩
-
4
On July 9, in an attempt to maintain control of BRCA testing, Myriad filed suit against two of the firms in a federal district court, arguing that both infringed the company’s multiple patents on such tests that were not challenged in the case. While the company’s stock price rallied, the legal merits of the suit were debatable. One of the companies promptly announced that it would vigorously defend its right to offer the tests. The ACLU considers Myriad’s action legally without merit and intends to file an amicus brief in support of the defendants. “The Supreme Court clearly rejected this monopolization of genetic information,” Sandra Park, an ACLU co-counsel in the Myriad case, notes in a recent e-mail to me.
In an evident gesture of conciliation to its critics, Myriad also declared that it would no longer seek to restrict noncommercial BRCA research and would permit other laboratories to offer independent BRCA tests for second opinions. See Andrew Pollack, “2 Competitors Sued by Genetics Company for Patent Infringement,” The New York Times, July 10, 2013. ↩ -
5
In fact, in July the Public Patent Foundation and another nonprofit group filed suit to nullify the basic patent on human stem cells in part because they are products of nature. See “US Stem Cell Patent Challenged,” Science, July 12, 2013. ↩




